Brii Biosciences Provides Corporate Updates and Reports 2023 Interim Results
First patient dosed in a PEG-IFN-α controlled BRII-835 + PEG-IFN-α combination Phase 2 study for HBV functional cure
First of several studies to investigate the potential of BRII-179 in enriching patients with strong intrinsic anti-HBsAg responses for curative treatments to start before the end of 2023
Regulatory submissions and preparation for launches of PreHevbri® in APAC countries and regions are underway
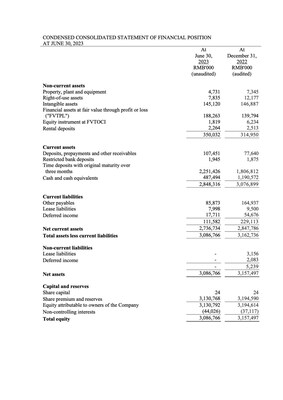
Near-term revenue opportunities with PreHevbri® and strong balance sheet supporting operations through 2026
Company to host earnings call on August 22 at 8:00 PM ET / August 23 at 8:00 AM HKT and an HBV R&D Day on August 24 from 1:00-2:30 PM HKT
DURHAM, N.C. and BEIJING, Aug. 22, 2023 /PRNewswire/ -- Brii Biosciences Limited ("Brii Bio," "we," or the "Company," stock code: 2137.HK), a biotechnology company developing therapies to improve patient health and choice across diseases with high unmet need, today announced a corporate update and reported its interim results for the first six months ended June 30, 2023.
Infectious Disease Therapeutic Area
Recently, Brii Bio executed two strategic transactions with VBI Vaccines Inc. (NASDAQ:VBIV), gaining exclusive global rights to BRII-179 and introducing PreHevbri®, a clinically differentiated prophylactic hepatitis B vaccine, to APAC countries and regions.
The addition of PreHevbri® complements Brii Bio's existing functional cure portfolio, further advancing solutions to reduce the transmission of HBV across Greater China and the other Asia Pacific countries and regions. The Company is actively working towards the preparation for launches of PreHevbri® in APAC markets, prioritizing countries and regions where additional trials may not be required. A Market Authorization Application has been filed in Hong Kong, and the Company expects a regulatory decision in the near future.
In June, Brii Bio's strategic partner, Vir Biotechnology, Inc. (NASDAQ:VIR), shared data from Part A of its Phase 2 MARCH trial of VIR-2218 (BRII-835) and VIR-3434 (BRII-877) at the 2023 European Association for the Study of the Liver (EASL) Congress, demonstrating significant declines in HBsAg levels and 90% achievement of HBsAg level below 10 IU/mL in chronic HBV participants, suggesting promising potential of VIR-3434 (BRII-877) in chronic HBV treatment. On the heels of these data, Brii Bio obtained Investigational New Drug (IND) approval from the Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) of China for a Phase 1 study of BRII-877 (VIR-3434) in August 2023.
Additionally, compelling data highlights the potential of BRII-835 (VIR-2218)/PEG-IFN-α combination as a best-in-class functional curative treatment for chronic HBV infections. Findings show that robust anti-HBs antibody responses at the end of treatment were associated with sustained HBsAg loss 24 weeks post-treatment, pointing to the important role of patients' humoral immunity in achieving sustained immune control of HBV infections.
Building upon this critical insight, Brii Bio has initiated a randomized and active-controlled BRII-835 + PEG-IFN-α Phase 2 study, following regulatory approvals from multiple regulatory authorities in APAC including the NMPA in mainland China. The primary objective of the study is to compare the functional cure rate of BRII-835/PEG-IFN-α combination versus PEG-IFN-α alone. Furthermore, the Company intends to include patients in the study who were previously exposed to BRII-179 and who had documented anti-HBsAg responses. The Company believes that BRII-179 has the unique ability to distinguish patients who have significant intrinsic humoral immunity versus those who do not. Planning of additional studies are also underway to investigate the role of BRII-179 as a primer to elicit stronger antibody responses and in enriching patients for curative treatments such as BRII-835/PEG-IFN-α as well as other combinations in broad HBV patient populations.
In a separate transaction with Qpex, Brii Bio acquired exclusive global rights to BRII-693 (also previously known as QPX9003), a potentially best-in-class synthetic lipopeptide IV antibiotic for combating difficult-to-treat multi-drug- and extremely-drug-resistant (MDR/XDR) gram-negative bacterial infections (especially carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa), reinforcing the Company's commitment to addressing antibiotic resistance challenges while strengthening financial support.
Central Nervous System Disease Therapeutic Area
Following an agreement with U.S. Food and Drug Administration, the Company will start a Phase 2 study of BRII-296 in postpartum depression (PPD) investigating the first-of-its-kind, long-acting, single treatment option in the third quarter of 2023. BRII-296 represents a paradigm shift in patient care with the potential to provide rapid and sustained relief of depressive symptoms for new mothers. It is estimated that approximately 500,000 mothers suffer from PPD in the U.S. alone, and globally, close to 20 million women are affected by PPD.
Additionally, the Company continues to advance a second long-acting injectable, BRII-297, in a first-in-human Phase 1 study, expanding the potentially groundbreaking treatment paradigm for various anxiety and depressive disorders or indications.
1H 2023 Financial Results
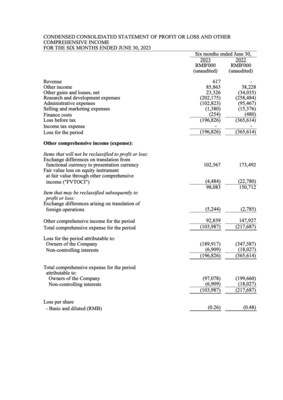
- Other Income was RMB85.9 million for 1H 2023, representing an increase of RMB47.7 million, or 124.9%, compared with RMB38.2 million for 1H 2022. The increase was mainly due to the increased bank interest income of RMB36.1 million attributable to the additional placement of time deposits with original maturity over three months and the increased income recognized from PRC government grants of RMB11.6 million.
- Research and development expenses were RMB202.2 million for 1H 2023, representing a decrease of RMB56.3 million, or 21.8%, compared with RMB258.5 million for 1H 2022. The decrease was primarily due to the reduced third-party contracting fees from COVID-19 programs after the Company decided to terminate these programs.
- Administrative expenses were RMB102.8 million for 1H 2023, representing an increase of RMB7.3 million, or 7.6%, compared with RMB95.5 million for 1H 2022. The increase was primarily attributable to the increase in employee headcounts and computer software fees.
- Total comprehensive expense for 1H 2023 was RMB104.0 million, representing a decrease of RMB113.7 million, or 52.2%, compared with RMB217.7 million for 1H 2022. The decrease was primarily due to the increase in other income and decrease in the research and development expenses.
Conference Call Information
A live conference call will be hosted on August 23, 2023, at 8:00 AM Hong Kong time (August 22, 2023, at 8:00 PM U.S. Eastern Time). To participate, please make sure to register in advance. For the registration link, please click here.
About Brii Bio
Brii Biosciences Limited ("Brii Bio", stock code: 2137.HK) is a commercial stage biotechnology company developing therapies to address major public health challenges where patients experience high unmet medical needs, limited choice and significant social stigmas. With a focus on infectious and central nervous system diseases, the Company is advancing a broad pipeline of unique therapeutic candidates with lead programs against hepatitis B viral infection (HBV), postpartum depression (PPD), and major depressive disorder (MDD). The Company is led by a visionary and experienced leadership team and has operations in key biotech hubs, including Raleigh-Durham, the San Francisco Bay Area, Beijing and Shanghai. For more information, visit www.briibio.com.
Forward Looking Statement
The information communicated in this press release contains certain statements that are or may be forward looking. These statements typically contain words such as "will," "expects," "believes," "plans" and "anticipates," and words of similar import. By their nature, forward looking statements involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. There may be additional material risks that are currently not considered to be material or of which the Company are unaware. These forward-looking statements are not a guarantee of future performance. Against the background of these uncertainties, readers should not rely on these forward-looking statements. The Company assumes no responsibility to update forward-looking statements or to adapt them to future events or developments.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/brii-biosciences-provides-corporate-updates-and-reports-2023-interim-results-301906916.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/brii-biosciences-provides-corporate-updates-and-reports-2023-interim-results-301906916.html
SOURCE Brii Biosciences Limited