Cognition Therapeutics Announces Results of Pre-specified Analysis of SHINE Study Data Presented at CTAD
- Results show CT1812 dramatically slowed cognitive decline in patients with lower levels of plasma p-tau217, an important biomarker of Alzheimer's disease pathology -
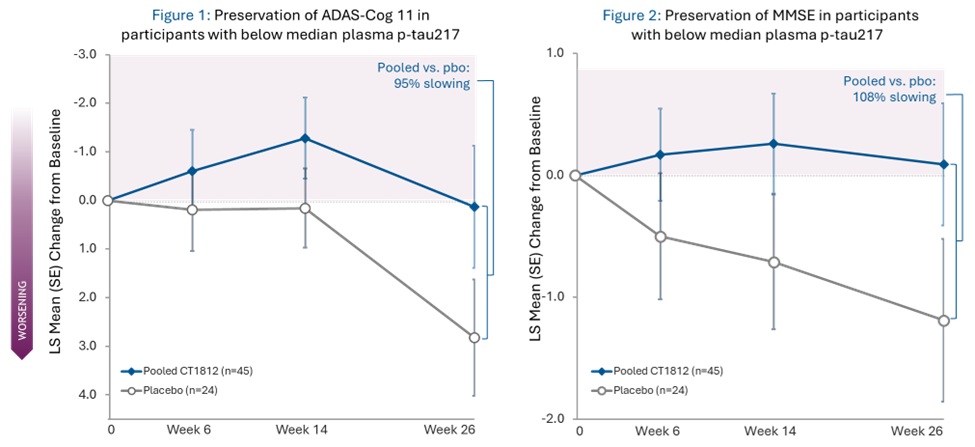
- ADAS-Cog 11 scores showed 95% slowing of decline -
- MMSE scores showed 108% slowing of decline -
- Pre-specified analysis from SHINE study presented by Dr. Michael Woodward at CTAD 2024 -
- Company hosting investor webinar on October 30, 2024 to review findings -
PURCHASE, N.Y., Oct. 29, 2024 (GLOBE NEWSWIRE) -- Cognition Therapeutics, Inc., (the "Company" or "Cognition") (NASDAQ:CGTX), a clinical-stage company developing drugs that treat neurodegenerative disorders, presented a pre-specified analysis of data from the Phase 2 SHINE study (NCT03507790) of CT1812 in participants with mild-to-moderate Alzheimer's disease today at the Clinical Trials on Alzheimer's Disease (CTAD) conference.
A 95% slowing of cognitive decline as measured by ADAS-Cog 11 was experienced by participants treated with CT1812 (pooled 100mg and 300mg) who had baseline plasma p-tau217 below the median (as shown in figure 1 below). When measured by MMSE, these same participants showed a 108% slowing (as shown in figure 2 below). Placebo patients in both analyses experienced cognitive decline.
p-Tau217 is an important biomarker that has shown the ability to distinguish Alzheimer's disease from other neurodegenerative disorders with a high degree of accuracy compared to other available biomarkers.*
"Participants with lower levels of p-tau217 who were treated with CT1812 performed extremely well in the SHINE study. These findings are consistent with the clinical experience of the anti-amyloid immunotherapeutics, which have been reported to be more effective in people with low p-tau," stated Michael Woodward, MD, associate professor, head of dementia research at Austin Health in Melbourne, Australia. "The degree of cognitive preservation in the CT1812-treated patients is impressive and could point to plasma p-tau217 as a predictive biomarker of robust treatment effect."
"The evidence Dr. Woodward presented at CTAD shows that CT1812 dramatically slowed cognitive decline in patients with lower levels of plasma p-tau217. This protein is an established biomarker of Alzheimer's disease pathology. It can be accurately detected using a commercially available blood test," added Anthony Caggiano, MD, PhD, CMO and head of R&D.
"In SHINE, we observed that the treatment effect size appears to be widening over time. We look forward to confirming this durable effect in a longer study. We believe that identifying patients with lower levels of plasma p-tau217 will enable us to enroll participants likely to experience the most robust treatment response. We look forward to discussing these results and potential approaches to Phase 3 with the FDA in an end-of-Phase 2 meeting," concluded Dr. Caggiano.
The CTAD presentation will be available on the Cognition Therapeutics website on the Publications page in accordance with the conference's embargo policy.
CTAD Presentation details
Title: Results from COG0201: a Randomized, Placebo-controlled, Double-blind, International, Phase 2 Study to Evaluate the Safety and Efficacy of CT1812 in Adults with Mild-to-Moderate Alzheimer's Disease
Authors: Woodward M, Vijverberg EGB, Di Caro V, Catalano S, Hamby ME, Grundman M, Iaci JF, Devins T, Caggiano AO
Date/Time: October 29, 2024: 5:55pm Central European Time
Location: Madrid, Spain
Webcast Information
Company management will present an overview of the results and discuss implications for the Company's clinical development plans for CT1812 at 8:00am ET on October 30, 2024. In addition, Anton Porsteinsson, MD of the University of Rochester Alzheimer's Disease Care, Research and Education Program will participate as an Alzheimer's industry expert. A live question and answer session will follow formal presentations.
Listening Options:
Participants can use the following guest dial-in numbers: 1-877-704-4453 or 1-201-389-0920 or select the Call me™ link for instant telephone access to the event Conference call. Call me™ will be made active 15 minutes prior to scheduled start time.
Alternatively, interested parties may register from the event listing on the Cognition Therapeutics Investor Relations webpage under Events or directly by visiting: https://viavid.webcasts.com/starthere.jsp?ei=1694880&tp_key=f312676b26. An archive of the webcast and presentation will be available beginning at approximately 11:00am ET on October 30, 2024.
About the SHINE Study
The SHINE study is a double-blind, placebo-controlled Phase 2 signal-finding trial that enrolled 153 adults with mild-to-moderate Alzheimer's disease who were evenly randomized to receive either placebo or one of two doses of CT1812 (100 mg or 300 mg), which was taken orally daily for six months. The primary endpoint was safety and tolerability. The key secondary endpoint of cognition was ADAS-Cog 11. Exploratory endpoints included change in MMSE, ADAS-Cog 13, ADCS-ADL and -CGIC as well as pre-specified subgroup analyses included a comparison of cognitive and functional changes in participants with plasma p-tau217 levels above and below the median. The SHINE study was supported by two grant awards from the National Institute on Aging of the National Institutes of Health (NIH) totaling approximately $30 million. More information may be found at clinicaltrials.gov under trial ID NCT03507790.
About CT1812
CT1812 is an experimental orally delivered small molecule oligomer antagonist that penetrates the blood-brain barrier and binds selectively to the sigma-2 (σ-2) receptor complex. Preclinical and clinical data demonstrate that this binding results in the displacement of toxic Aβ oligomers. The σ-2 receptor complex is involved in the regulation of key cellular processes such as membrane trafficking and autophagy that are damaged by toxic interaction with Aβ oligomers, oxidative stress and other stressors. This damage to sensitive synapses can progress to a loss of synaptic function, which manifests as cognitive impairment and Alzheimer's disease progression.
Participants are currently being recruited in the START study (NCT05531656) of CT1812 in adults with early Alzheimer's disease; and the MAGNIFY study (NCT05893537) in adults with geographic atrophy (GA) secondary to dry age-related macular degeneration. Enrollment has completed in the SHIMMER study (NCT05225415) of CT1812 in adults with dementia with Lewy bodies and the aforementioned SHINE Study.
About Cognition Therapeutics, Inc.
Cognition Therapeutics, Inc., is a clinical-stage biopharmaceutical company discovering and developing innovative, small molecule therapeutics targeting age-related degenerative disorders of the central nervous system and retina. We currently are investigating our lead candidate CT1812 in clinical programs in Alzheimer's disease, dementia with Lewy bodies (DLB) and dry age-related macular degeneration (dry AMD). We believe CT1812 and our pipeline of σ-2 receptor modulators can regulate pathways that are impaired in these degenerative diseases, with an approach that is functionally distinct from other treatments. More about Cognition Therapeutics and our pipeline can be found at https://cogrx.com.
* Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020;324(8):772–781.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements contained in this press release or made during the conference, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding our product candidates, including CT1812, and any expected or implied benefits or results, including that initial clinical results observed with respect to CT1812 will be replicated in later trials and our clinical development plans, including statements regarding our clinical studies of CT1812 and any analyses of the results therefrom, are forward-looking statements. These statements, including statements relating to the timing and expected results of our clinical trials involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as "may," "might," "will," "should," "expect," "plan," "aim," "seek," "anticipate," "could," "intend," "target," "project," "contemplate," "believe," "estimate," "predict," "forecast," "potential" or "continue" or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and results of operations. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: competition; our ability to secure new (and retain existing) grant funding; our ability to grow and manage growth, maintain relationships with suppliers and retain our management and key employees; our ability to successfully advance our current and future product candidates through development activities, preclinical studies and clinical trials and costs related thereto; uncertainties inherent in the results of preliminary data, pre-clinical studies and earlier-stage clinical trials being predictive of the results of early or later-stage clinical trials; the timing, scope and likelihood of regulatory filings and approvals, including regulatory approval of our product candidates; changes in applicable laws or regulations; the possibility that the we may be adversely affected by other economic, business or competitive factors, including ongoing economic uncertainty; our estimates of expenses and profitability; the evolution of the markets in which we compete; our ability to implement our strategic initiatives and continue to innovate our existing products; our ability to defend our intellectual property; the impacts of ongoing global and regional conflicts on our business, supply chain and labor force; and the risks and uncertainties described more fully in the "Risk Factors" section of our annual and quarterly reports filed with the Securities & Exchange Commission and are available at www.sec.gov. These risks are not exhaustive and we face both known and unknown risks. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
| Contact Information: Cognition Therapeutics, Inc. [email protected] | Casey McDonald (media) Tiberend Strategic Advisors, Inc. [email protected] | Mike Moyer (investors) LifeSci Advisors [email protected] | ||
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ea54fdce-dee9-447e-9730-effc2c45a211

