PureTech's Deupirfenidone (LYT-100) Demonstrates Strong and Durable Efficacy as a Monotherapy with Favorable Tolerability in Phase 2b ELEVATE IPF Trial
Deupirfenidone 825 mg TID slowed lung function decline in people with idiopathic pulmonary fibrosis (IPF) to the range expected of healthy older adults over 6 months; new, preliminary open-label extension data support durability of this treatment effect over at least 52 weeks
Deupirfenidone 825 mg TID demonstrated a statistically significant benefit compared to placebo in delaying IPF progression
Detailed safety analysis underscores favorable tolerability profile for deupirfenidone
PureTech plans to meet with FDA before end of Q3 2025, with the goal of initiating a Phase 3 trial by year-end
PureTech Health plc (NASDAQ:PRTC, LSE: PRTC)) ("PureTech" or the "Company"), a clinical-stage biotherapeutics company dedicated to changing the lives of patients with devastating diseases, delivered a late-breaking, oral presentation at the 2025 American Thoracic Society (ATS) International Conference in San Francisco. The presentation provided further insights into the successful Phase 2b ELEVATE IPF trial of deupirfenidone (LYT-100), highlighting the strength and durability of deupirfenidone's treatment effect through at least 52 weeks while maintaining favorable tolerability in patients living with idiopathic pulmonary fibrosis (IPF).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250520345638/en/
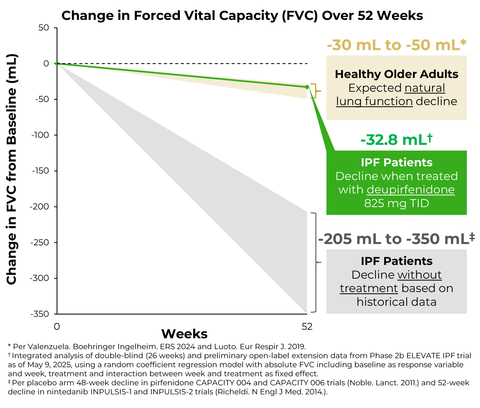
Preliminary data (as of May 9, 2025) from the ongoing open-label extension (OLE) suggest that the rate of FVC decline observed in the double-blind portion of the trial over 26 weeks with deupirfenidone 825 mg TID (-21.5 mL) is durable out to at least 52 weeks. Those in the deupirfenidone 825 mg TID arm experienced a decline in FVC of -32.8 mL over the 52-week period, which is similar to the expected natural decline in lung function in healthy older adults over one year (approximately -30.0 mL to -50.0 mL).
"The ELEVATE IPF trial is one of the most promising Phase 2 studies we've seen in IPF in recent years," said Toby Maher, M.D., Ph.D., Professor of Medicine and Director of Interstitial Lung Disease at Keck School of Medicine, University of Southern California, Los Angeles, and lead investigator in the ELEVATE IPF trial. "The ability for a monotherapy to reduce lung function decline close to a level seen in healthy older adults, and to sustain that effect over time without compromising tolerability, is not something we have seen with currently available therapies. Deupirfenidone has the potential to raise the bar for what patients and physicians can expect from IPF treatment."
Data presented from PureTech's global Phase 2b randomized, double-blind, active- and placebo-controlled, dose-ranging ELEVATE IPF trial demonstrated the potential for deupirfenidone to offer a differentiated treatment option for patients with IPF. In the trial, patients treated with deupirfenidone 825 mg three times a day (TID) experienced a slower rate of lung function decline,1 as measured by Forced Vital Capacity (FVC), at 26 weeks versus those who were treated with placebo (-21.5 mL vs. -112.5 mL, respectively; p=0.02).2 This statistically significant difference represents a robust treatment effect versus placebo of 80.9% for deupirfenidone 825 mg TID as a monotherapy. This result compares favorably against the rate of decline in FVC observed in the trial among patients treated with pirfenidone 801 mg TID versus placebo (-51.6 mL vs. -112.5 mL, respectively), which was consistent with previously reported pirfenidone clinical trial data3 and represents a treatment effect of 54.1%. Taken together, these results indicate that the treatment effect with deupirfenidone 825 mg TID was approximately 50% greater than that of pirfenidone 801 mg TID, based on their respective reductions in lung function decline versus placebo (80.9% vs. 54.1%).
In addition to these findings, deupirfenidone 825 mg TID also demonstrated a statistically significant benefit in delaying time to IPF progression4 compared to placebo (hazard ratio = 0.439; p=0.0023), further supporting the clinical relevance of the treatment effect.
Importantly, the rate of FVC decline observed over 26 weeks with deupirfenidone 825 mg TID (-21.5 mL) was similar to the expected natural decline in lung function in healthy older adults (approximately -15.0 mL to -25.0 mL).5,6 Furthermore, preliminary data from the ongoing open-label extension (OLE) study suggest that this treatment effect is durable out to at least 52 weeks. As of May 9, 2025, a total of 101 patients had received at least 52 weeks of treatment with deupirfenidone. Those in the deupirfenidone 825 mg TID arm experienced a decline in FVC of -32.8 mL over the 52-week period,7 which is similar to the expected natural decline in lung function in healthy older adults over one year (approximately -30.0 mL to -50.0 mL).6 These new data provide additional support for the durability of the treatment effect observed with this dose and reinforce its potential to stabilize lung function decline over time, while maintaining favorable safety and tolerability. Additional details from the ongoing OLE are expected to be shared in a future scientific forum.
These results are further supported by preliminary pharmacokinetic (PK) data, which underscore the differentiated profile of deupirfenidone. Compared to pirfenidone 801 mg TID, deupirfenidone 825 mg TID resulted in an approximately 50% increase in drug exposure. Notably, the dramatically increased drug exposure did not result in an increase in tolerability challenges, suggesting that the deuterated structure of deupirfenidone may overcome the dose-limiting adverse events associated with pirfenidone. PureTech believes these PK results are consistent with the enhanced efficacy and favorable tolerability seen with deupirfenidone 825 mg TID in the trial.
"The IPF community has long needed therapies that can provide meaningful efficacy without compromising tolerability," said Bharatt Chowrira, Ph.D., J.D., Chief Executive Officer of PureTech. "Data from our Phase 2b trial and open-label extension study suggest that deupirfenidone may slow lung function decline in a way that more closely mirrors the natural aging process, and that this effect is durable. These data are quite remarkable and – to our knowledge – this level of efficacy has not been observed with other monotherapies. These findings further support our belief that deupirfenidone may offer a substantially differentiated treatment option for people living with IPF and support its potential to become a new standard of care."
Deupirfenidone was well tolerated at both doses studied. Safety analyses included identification of the 16 most common treatment-emergent adverse events (TEAEs), defined as occurring in more than 5% of participants in at least one treatment group, and characterized the arm with the highest relative incidence of each of these 16 TEAEs. The pirfenidone 801 mg treatment group had the highest relative incidence for 9 of these TEAEs, followed by deupirfenidone 825 mg (5), placebo (2), and deupirfenidone 550 mg (0).
"The results of the ELEVATE IPF trial demonstrate the potential for deupirfenidone to address the persistent suboptimal efficacy offered by current standard-of-care treatments for IPF, without sacrificing tolerability," Camilla Graham, M.D., M.P.H., Senior Vice President of Medical Affairs at PureTech. "We are excited to continue development of deupirfenidone to meaningfully improve the lives of patients living with IPF."
PureTech is targeting a meeting with the U.S. Food and Drug Administration by the end of the third quarter of 2025 to discuss the results of the Phase 2b trial and align on a potential registrational pathway, with the goal of initiating a Phase 3 trial by the end of 2025. PureTech anticipates providing further guidance later this year following the finalization of the trial design and FDA interactions.
About the ELEVATE IPF Trial
The Phase 2b ELEVATE IPF trial was a global, randomized, double-blind, active- and placebo-controlled, dose-ranging trial designed to evaluate the efficacy, tolerability, safety and dosing regimen of deupirfenidone (LYT-100) in patients with IPF compared to placebo. 257 participants were randomized in a ratio of 1:1:1:1 to receive either 550 mg of deupirfenidone, 825 mg of deupirfenidone, 801 mg pirfenidone or placebo three times a day (TID) for 26 weeks. Participants who completed the trial had the option to enroll in an open-label extension, which is ongoing.
The primary endpoint of the trial was the rate of decline in Forced Vital Capacity (FVC) for the combined deupirfenidone arms versus placebo over the 26-week treatment period. FVC is a measure of the maximum amount of air (in mL) that an individual can forcibly exhale after fully inhaling. It is a standard measurement in clinical trials for IPF and is used to assess disease progression as well as to predict mortality.
A prespecified Bayesian analysis was utilized to assess the primary endpoint and provided a posterior probability, which is the probability of superior efficacy for deupirfenidone compared to placebo. This also allowed for augmentation of the placebo arm with placebo data from historical IPF trials. This approach enabled a more patient-centric clinical trial design by minimizing the number of trial participants exposed to placebo – a key consideration since IPF is progressive and fatal – while delivering a robust, placebo-controlled dataset.
About Deupirfenidone (LYT-100)
Deupirfenidone (LYT-100) is an investigational therapy in development as a potential new standard of care (SOC) for the treatment of idiopathic pulmonary fibrosis (IPF). It is a deuterated form of pirfenidone, which – along with nintedanib – is one of the two FDA-approved treatments for IPF. Despite achieving blockbuster status, the current SOC treatments only modestly slow lung function decline, with tolerability limiting the ability to achieve higher doses. This results in suboptimal efficacy, reduced patient uptake, and poor adherence – all due to a tolerability ceiling that prevents dosing levels that could significantly improve patient outcomes.
Deupirfenidone may overcome these limitations. In the global Phase 2b ELEVATE IPF trial, deupirfenidone demonstrated the potential to stabilize lung function decline over at least 26 weeks as a monotherapy while maintaining safety and tolerability – a result not previously achieved by other investigational or marketed IPF therapies to the Company's knowledge. These findings support the potential for deupirfenidone to offer a meaningful advance for patients living with this progressive and life-limiting disease. Beyond IPF, deupirfenidone may also address multiple underserved fibrotic diseases, including progressive fibrosing interstitial lung diseases and other fibrotic conditions.
About Idiopathic Pulmonary Fibrosis (IPF)
Idiopathic Pulmonary Fibrosis (IPF) is a rare, progressive and fatal lung disease characterized by irreversible scarring of lung tissue. Median survival following diagnosis is estimated to be two to five years.8 IPF affects more than 230,000 people across the United States and EU5 (France, Germany, Italy, Spain, and the United Kingdom).9
Although two therapies are approved to treat IPF, their use remains limited, and nearly three out of four people with IPF in the United States have never received either treatment.10 There remains a significant need for therapies that can more effectively slow or stabilize disease progression, while maintaining favorable tolerability, to improve outcomes for people living with IPF.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to giving life to new classes of medicine to change the lives of patients with devastating diseases. The Company has created a broad and deep portfolio through its experienced research and development team and its extensive network of scientists, clinicians, and industry leaders that is being advanced both internally and through its Founded Entities. PureTech's R&D engine has resulted in the development of 29 therapeutics and therapeutic candidates, including three that have been approved by the U.S. Food and Drug Administration. A number of these programs are being advanced by PureTech or its Founded Entities in various indications and stages of clinical development, including registration-enabling studies. All of the underlying programs and platforms that resulted in this portfolio of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points.
For more information, visit www.puretechhealth.com or connect with us on X (formerly Twitter) @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation those related to the deupirfenidone (LYT-100) development program and development plans, its potential benefits to patients, plans for discussions with regulatory authorities, the further development of the program, future presentation of additional data from the trial and our future prospects, developments and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption "Risk Factors" in our Annual Report on Form 20-F for the year ended December 31, 2024, filed with the SEC and in our other regulatory filings. These forward-looking statements are based on assumptions regarding the present and future business strategies of the Company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, we disclaim any obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
References 1 Efficacy analysis used a random coefficient regression model with absolute FVC including baseline as response variable and week, treatment and interaction between week and treatment as fixed effect. The analysis was performed based on the predefined Full Analysis Set. 2 All p values are two-sided and have not been corrected for multiplicity. 3 Roche. (2014). Esbriet® (pirfenidone) prescribing information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf 4 IPF progression was defined as a ≥5% decline in FVCpp or death. 5 FVC decline at 6 months was estimated assuming linear decline over time. 6 Valenzuela, C., Bonella, F., Moor, C., Weimann, G., Miede, C., Stowasser, S., & Maher, T. (2024, September). Decline in forced vital capacity (FVC) in subjects with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) compared with healthy references [Poster presentation]. European Respiratory Society International Congress, Vienna, Austria; and Luoto, J., Pihlsgård, M., Wollmer, P., & Elmståhl, S. (2019). Relative and absolute lung function change in a general population aged 60–102 years. European Respiratory Journal, 53(3), 1701812. https://doi.org/10.1183/13993003.01812-2017 |
View source version on businesswire.com: https://www.businesswire.com/news/home/20250520345638/en/
PureTech
Public Relations
[email protected]
Investor Relations
[email protected]
UK/EU Media
Ben Atwell, Rob Winder
+44 (0) 20 3727 1000
[email protected]
US Media
Justin Chen
+1 609 578 7230
[email protected]
